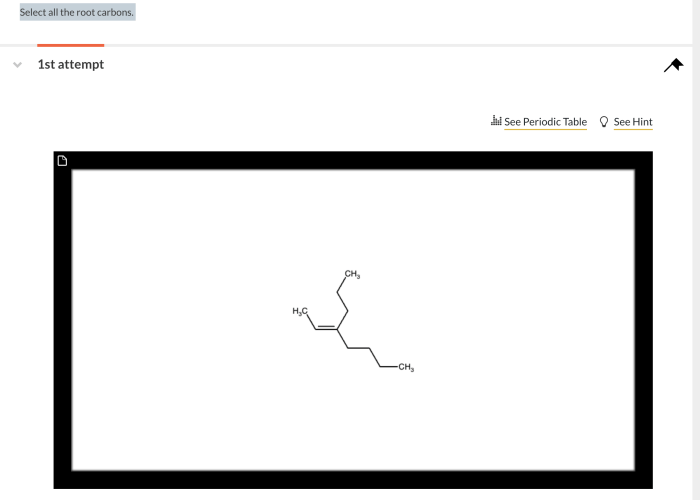
Select all the root carbons. This seemingly simple task is a fundamental aspect of organic chemistry, providing a deeper understanding of molecular structure and reactivity. Delving into the realm of root carbons, we’ll uncover their definition, identification methods, properties, and significance in organic reactions and beyond.
The identification of root carbons serves as a cornerstone in comprehending the behavior of organic molecules. By mastering this skill, chemists gain a powerful tool to decipher reaction mechanisms, predict product formation, and harness the potential of organic compounds in various fields.
Root Carbons Definition
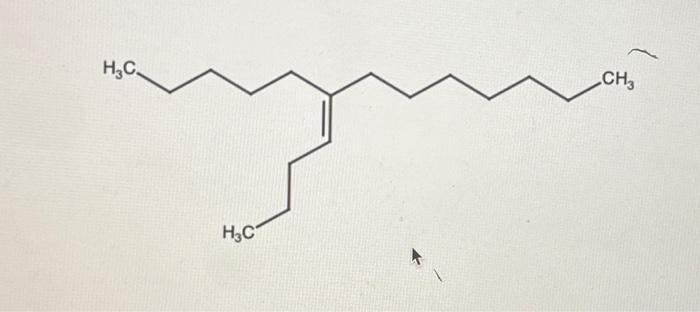
Root carbons are the carbons in an organic molecule that are directly connected to the functional group. They are the most important carbons in the molecule because they determine the chemical reactivity of the molecule.
For example, in the molecule CH3CH2OH, the root carbon is the carbon that is bonded to the oxygen atom. This carbon is the most reactive carbon in the molecule because it is the most likely to be attacked by other molecules.
Identifying Root Carbons
To identify the root carbons in an organic molecule, you need to first identify the functional group. Once you have identified the functional group, the root carbons are the carbons that are directly connected to the functional group.
Identifying the root carbons is crucial in organic chemistry. It’s like the foundation of a house – everything else builds upon it. But hey, if you’re looking for some literary inspiration, check out prayer for owen meany quotes . They’re like poetic blueprints, guiding us through the complexities of life.
Anyway, back to our carbons – they’re the key to understanding the structure and properties of organic molecules.
Here are some examples of root carbons:
- In the molecule CH3CH2OH, the root carbon is the carbon that is bonded to the oxygen atom.
- In the molecule CH3COOH, the root carbon is the carbon that is bonded to the carboxyl group.
- In the molecule CH3NH2, the root carbon is the carbon that is bonded to the amino group.
Identifying Root Carbons
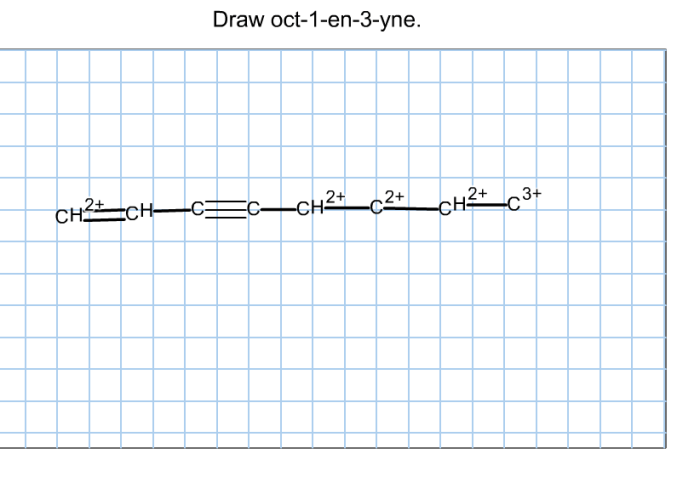
Identifying root carbons is a crucial step in understanding the structure and properties of organic molecules. Root carbons form the backbone of the molecule and provide a reference point for other atoms and functional groups. Here are the methods commonly used to identify root carbons:
- Connectivity:Root carbons are typically connected to the most atoms in the molecule. They often form the central core or framework of the molecule.
- Degree of Substitution:Root carbons have the highest degree of substitution among the carbons in the molecule. They are typically bonded to four other atoms or groups.
- Hybridization:Root carbons are usually sp3 hybridized, meaning they have four electron pairs arranged in a tetrahedral geometry.
Example:In the molecule CH3CH2CH2CH3, the root carbons are the two middle carbons (C2 and C3). These carbons are connected to four other atoms and have a tetrahedral geometry. Practice Exercise:Identify the root carbons in the following molecules:
- CH3CH(OH)CH3
- CH3CH2CH(CH3)CH2CH3
- C6H6
Properties of Root Carbons
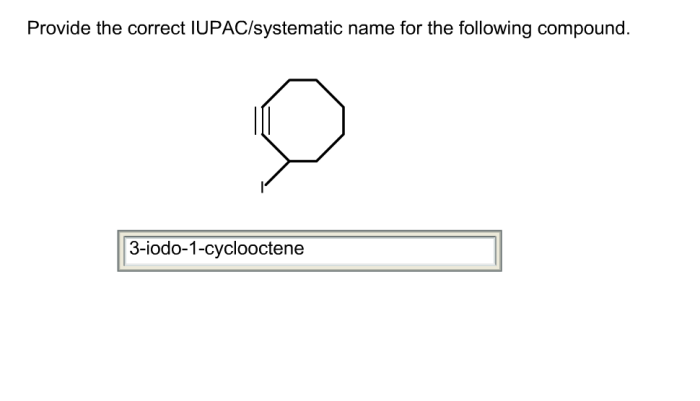
Root carbons possess unique chemical properties and characteristics that distinguish them from other types of carbon atoms in organic molecules.
These properties include:
Reactivity
- Root carbons are generally less reactive than other types of carbon atoms due to their high degree of substitution.
- They are less likely to undergo electrophilic addition or substitution reactions.
Stability
- Root carbons are more stable than other types of carbon atoms due to their high degree of substitution.
- They are less likely to undergo rearrangement or fragmentation reactions.
Other Properties, Select all the root carbons.
- Root carbons are typically sp3 hybridized, meaning they have four single bonds to other atoms.
- They are often found in the interior of organic molecules, away from the periphery.
Root Carbons in Organic Reactions: Select All The Root Carbons.
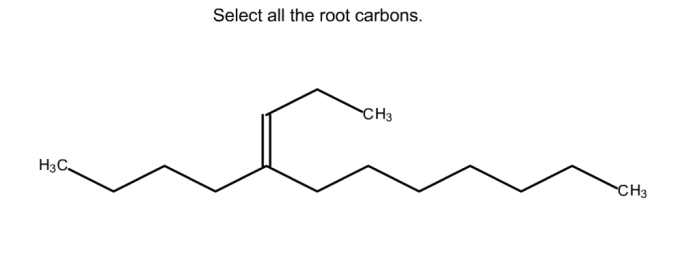
Root carbons play crucial roles in various organic reactions, influencing the reaction mechanisms and product outcomes. Their unique structural features enable them to participate in a range of chemical transformations.
Participation in Nucleophilic Reactions
Root carbons serve as electrophilic centers in nucleophilic reactions. They readily react with nucleophiles, such as hydroxide ions or Grignard reagents, to form new carbon-carbon bonds. This reactivity is attributed to the positive charge on the root carbon, which attracts electron-rich nucleophiles.
Participation in Electrophilic Reactions
Root carbons can also participate in electrophilic reactions, where they act as nucleophiles. They react with electrophiles, such as protons or alkyl halides, to form new carbon-carbon or carbon-heteroatom bonds. The presence of the positive charge on the root carbon facilitates the nucleophilic attack on electrophiles.
Involvement in Radical Reactions
Root carbons are prone to radical reactions due to their high reactivity. They can undergo homolytic bond cleavage to form free radicals, which can participate in a variety of radical reactions, such as radical additions, radical substitutions, and radical rearrangements.
Examples of Root Carbon Involvement in Reactions
- In the Friedel-Crafts alkylation reaction, the root carbon of an alkyl halide reacts with an electrophile (e.g., an aromatic ring) to form a new carbon-carbon bond.
- In the Grignard reaction, the root carbon of a Grignard reagent reacts with an electrophile (e.g., a ketone or aldehyde) to form a new carbon-carbon bond.
- In the free radical chlorination of alkanes, the root carbon of an alkane undergoes homolytic bond cleavage to form a free radical, which then reacts with a chlorine radical to form a chlorinated alkane.
Applications of Root Carbon Knowledge
Understanding root carbons has practical applications in various fields. They play a crucial role in drug design and materials science.
Drug Design
Root carbons provide valuable insights into the structure-activity relationships of drug molecules. By identifying root carbons, researchers can design drugs with enhanced potency, selectivity, and reduced side effects. This knowledge aids in the development of more effective and targeted therapies.
Materials Science
In materials science, root carbons influence the properties of materials such as polymers, plastics, and ceramics. Understanding root carbons allows scientists to tailor the mechanical, electrical, and thermal properties of materials, leading to the development of advanced materials with desired functionalities.
Popular Questions
What are root carbons?
Root carbons are the carbons in an organic molecule that are directly attached to the functional group.
How do I identify root carbons?
To identify root carbons, locate the functional group in the molecule and identify the carbons that are directly bonded to it.
What is the importance of root carbons?
Root carbons play a crucial role in determining the reactivity and properties of organic molecules.