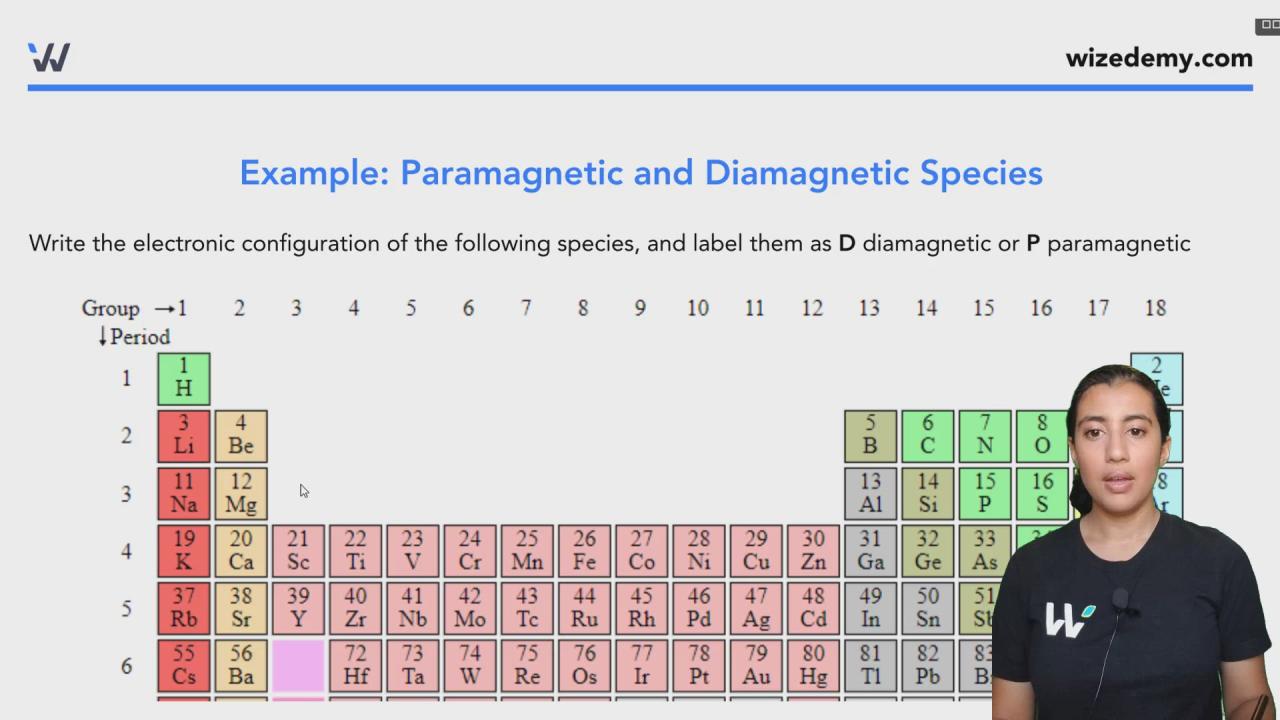
Is c2+ paramagnetic or diamagnetic – As we delve into the intriguing realm of chemistry, we encounter C2+, a species that holds the key to unlocking the secrets of magnetism. Embarking on a journey to unravel its magnetic nature, we will explore the fundamental principles that govern paramagnetism and diamagnetism, unraveling the intricate interplay between electronic configurations and magnetic properties.
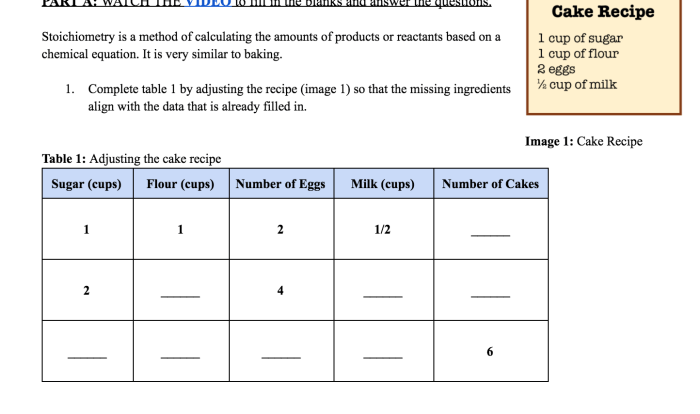
Join us as we illuminate the captivating world of C2+, where the dance of electrons orchestrates its magnetic symphony.
The electronic configuration of C2+ plays a pivotal role in shaping its magnetic behavior. Delving into the quantum realm, we will dissect the distribution of electrons within its orbitals, uncovering how this arrangement dictates whether C2+ aligns with or opposes an applied magnetic field.
Along the way, we will encounter experimental techniques that allow us to probe the magnetic properties of substances, unraveling the mysteries that lie within their atomic structures.
Chemical Properties of C2+
The chemical properties of C2+ are primarily determined by its electronic configuration and the resulting molecular structure. Understanding these properties is crucial for comprehending the magnetic nature of C2+.
C2+ exists as a diatomic molecule with a carbon-carbon triple bond. The electronic configuration of each carbon atom in C2+ is 1s 22s 12p 2. When these two carbon atoms form a triple bond, they share six electrons, resulting in a molecular orbital configuration of σ 1s2σ 1s* 2σ 2s2σ 2s* 2π 2p2π 2p* 2.
Bonding and Molecular Structure
The triple bond in C2+ consists of one sigma bond (σ) and two pi bonds (π). The σ bond is formed by the overlap of the 2s orbitals of the two carbon atoms, while the π bonds are formed by the overlap of the 2p orbitals.
The presence of two π bonds in C2+ gives rise to its unique magnetic properties.
Unpaired Electrons
In the molecular orbital configuration of C2+, the π 2p* 2orbital is partially filled, containing two unpaired electrons. These unpaired electrons are responsible for the paramagnetic nature of C2+.
Paramagnetism and Diamagnetism: Is C2+ Paramagnetic Or Diamagnetic
In the realm of magnetism, substances can exhibit contrasting behaviors when exposed to an external magnetic field. These behaviors are classified as paramagnetism and diamagnetism, which stem from the inherent magnetic properties of their constituent atoms or molecules.
Paramagnetism
Paramagnetism arises when a substance contains unpaired electrons, which possess intrinsic magnetic moments. These unpaired electrons align with the applied magnetic field, causing the substance to become weakly attracted to the field. Examples of paramagnetic substances include oxygen, aluminum, and transition metals.
Diamagnetism
Diamagnetism, on the other hand, is exhibited by substances with all electrons paired. In such substances, the application of a magnetic field induces a weak opposing magnetic field within the material. This results in a slight repulsion from the applied field.
Examples of diamagnetic substances include helium, copper, and most organic compounds.
Factors Determining Paramagnetism and Diamagnetism
The paramagnetic or diamagnetic nature of a substance is primarily determined by the number of unpaired electrons it possesses. Substances with unpaired electrons are paramagnetic, while those with all electrons paired are diamagnetic.
Experimental Techniques for Determining Magnetic Properties
Determining the magnetic properties of substances is crucial for understanding their electronic structure and behavior. Various experimental techniques have been developed to measure and characterize these properties.
These techniques rely on the interaction between the substance’s magnetic moments and an applied magnetic field. By analyzing the response of the substance to the field, researchers can determine its magnetic susceptibility, a measure of its ability to become magnetized.
Magnetic Susceptibility Measurements
Magnetic susceptibility measurements are commonly used to determine the magnetic properties of substances. These measurements involve placing the sample in a known magnetic field and measuring the force exerted on it.
The force experienced by the sample is proportional to its magnetic susceptibility, which can be positive (paramagnetic) or negative (diamagnetic). Paramagnetic substances have unpaired electrons that align with the applied field, while diamagnetic substances have all electrons paired and experience a weak repulsion from the field.
Electron Paramagnetic Resonance (EPR) Spectroscopy
Electron paramagnetic resonance (EPR) spectroscopy is a technique used to study paramagnetic substances. It involves exposing the sample to a microwave field in the presence of a magnetic field.
When the energy of the microwave field matches the energy difference between the two electron spin states, resonance occurs, and the sample absorbs energy. The EPR spectrum provides information about the number of unpaired electrons, their spin state, and the interactions between them.
Applications to C2+
The magnetic properties of C2+ can be determined using the techniques described above. Magnetic susceptibility measurements indicate that C2+ is paramagnetic, as it has one unpaired electron.
EPR spectroscopy can further characterize the electronic structure of C2+ and provide information about the spin state of the unpaired electron. This information can be used to understand the bonding and reactivity of C2+ in various chemical environments.
Applications of Magnetic Properties
Magnetic properties play a crucial role in various fields of science and technology. The magnetic properties of C2+ can be utilized in practical applications, such as:
Medical Imaging, Is c2+ paramagnetic or diamagnetic
Magnetic resonance imaging (MRI) is a medical imaging technique that uses the magnetic properties of atoms to create detailed images of the inside of the body. C2+ can be used as a contrast agent in MRI, which enhances the visibility of certain tissues and organs.
Chemical Analysis
Electron paramagnetic resonance (EPR) spectroscopy is a technique used to study the magnetic properties of materials. EPR spectroscopy can be used to identify and characterize free radicals, which are molecules with unpaired electrons. C2+ can be used as a probe in EPR spectroscopy to study the magnetic properties of other materials.
Magnetic Materials
The magnetic properties of C2+ can be used to create new types of magnetic materials. These materials could have applications in data storage, sensors, and other electronic devices.
Future Applications
The magnetic properties of C2+ are still being explored, and there is potential for future applications in a variety of fields. For example, C2+ could be used to create new types of batteries or fuel cells.
Top FAQs
What is the significance of C2+’s electronic configuration in determining its magnetic nature?
The electronic configuration of C2+ dictates the number of unpaired electrons it possesses. Unpaired electrons are responsible for the magnetic moment of a substance, and their presence or absence determines whether C2+ exhibits paramagnetic or diamagnetic behavior.
How can experimental techniques be used to determine the magnetic properties of C2+?
Experimental techniques such as the Gouy method and the Faraday method can be employed to measure the magnetic susceptibility of C2+, providing insights into its magnetic behavior. These techniques allow us to quantify the extent to which C2+ is attracted to or repelled by a magnetic field.
What are some potential applications of C2+’s magnetic properties?
The magnetic properties of C2+ could find applications in the development of magnetic materials, sensors, and catalysts. Its unique magnetic behavior could also be harnessed for energy storage and conversion technologies.